Are you wanting to find 'polysiloxanes synthesis structure'? All the details can be found here.
The basic structural units of polysiloxanes ar Chain polysiloxanes ar composed of difunctional units. The framework groups (R) ar either H operating room organic moieties and the end groups are usually Operating theater or a monofunctional siloxyl unit. Chain polysiloxanes such equally poly- (dimethylsiloxane)s ar synthesized by the hydrolysis of dichlorodimethysilane [ Eq.
Table of contents
- Polysiloxanes synthesis structure in 2021
- Polysiloxane properties
- Polysiloxane polymer
- Polysiloxane glass transition temperature
- Polysiloxane structure
- Polysiloxanes silicon dioxide
- Polysiloxane medical use
- Polysiloxane chemical formula
Polysiloxanes synthesis structure in 2021
 This picture illustrates polysiloxanes synthesis structure.
This picture illustrates polysiloxanes synthesis structure.
Polysiloxane properties
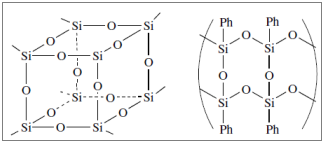 This picture shows Polysiloxane properties.
This picture shows Polysiloxane properties.
Polysiloxane polymer
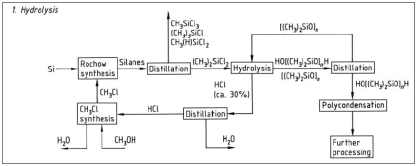 This picture demonstrates Polysiloxane polymer.
This picture demonstrates Polysiloxane polymer.
Polysiloxane glass transition temperature
 This picture illustrates Polysiloxane glass transition temperature.
This picture illustrates Polysiloxane glass transition temperature.
Polysiloxane structure
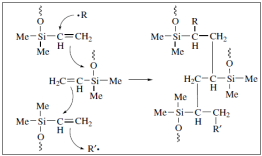 This picture representes Polysiloxane structure.
This picture representes Polysiloxane structure.
Polysiloxanes silicon dioxide
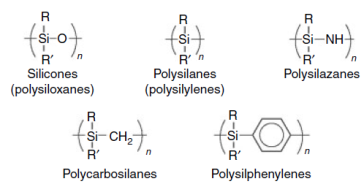 This picture illustrates Polysiloxanes silicon dioxide.
This picture illustrates Polysiloxanes silicon dioxide.
Polysiloxane medical use
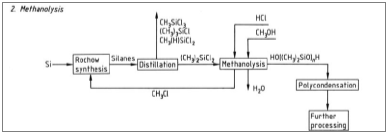 This image demonstrates Polysiloxane medical use.
This image demonstrates Polysiloxane medical use.
Polysiloxane chemical formula
What makes up the main chain of a polysiloxane?
Polysiloxanes are considered as inorganic – organic hybrid materials. The main chain is comprised of atoms of Si and O, whereas the side groups are comprised of alkyl groups (figure 2). Figure 2: Structure of a polysiloxane.
How are silicone oil and polysiloxanes synthesized?
Silicone oils are linear polymers synthesized by ring – opening polymerization. They are thermally stable and as a result they can be used as heat transfer fluids. Elastomer silicones are cross linked polysiloxanes via vulcanization and can be used in cars and food containers.
How are polysiloxanes used in everyday life?
Figure 2: Structure of a polysiloxane. This structure is enough to grant them with excessive properties, thus they can be used in many different applications in our everyday life. These polymers can be found as silicone oils, elastomers and resins. Silicone oils are linear polymers synthesized by ring – opening polymerization.
Who was the first person to make polysiloxanes?
The first polysiloxanes were synthesized by F. S. Kipping in the beginning of the twentieth century. Kipping synthesized diorgano – dichloro – silanes, R 2 SiCl 2, that could be hydrolyzed into R 2 Si (OH) 2. He was expecting that if these compounds got dehydrated they would produce compounds similar to ketones, R 2 Si = O.
Last Update: Oct 2021